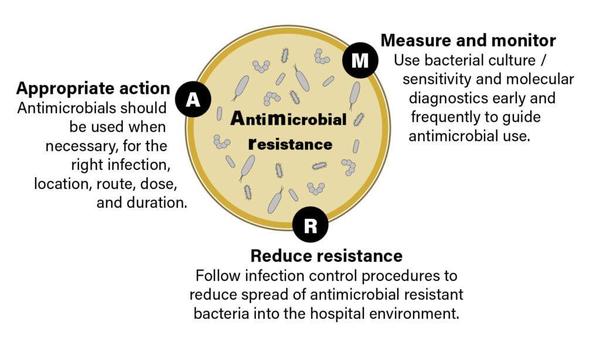
Photo by MART PRODUCTION on Pexels
![]()


Biomedical Science postgraduate with a strong foundation in medical communications, regulatory writing, clinical trials and hands-on clinical experience.

Hi, I’m Yash - a Biomedical Science graduate with a P.G.Dip. in Clinical Studies, Medical Writing and Data Management. I specialize in regulatory writing, clinical study documentation and translating complex scientific data into clear, accurate content. My goal is to support healthcare and research by creating precise, evidence-based materials for clinicians, researchers and regulatory stakeholders.